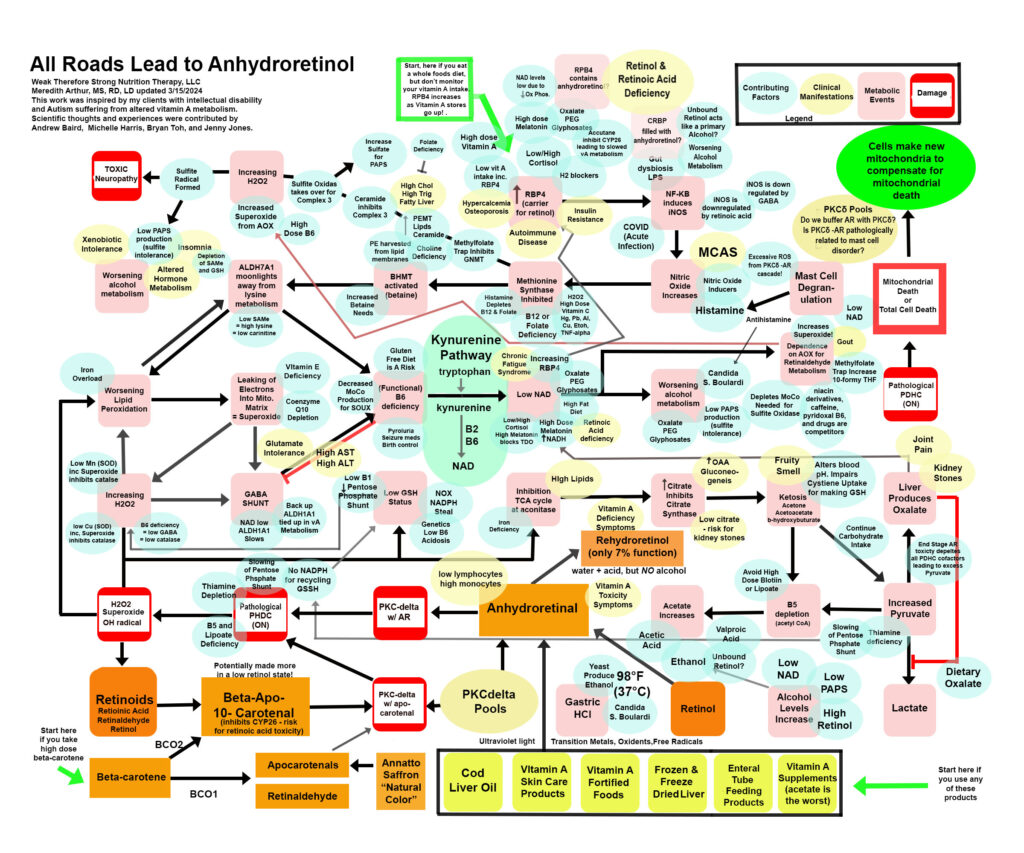
Anhydroretinol (AR) is a metabolic byproduct of vitamin A degradation as well as vitamin A metabolism. We can consume it in varying amounts in our diet, and our body, in the absence of a primary alcohol, will convert AR to rehydroretinol using acid and water. Rehydroretinol only has 7% of the function of retinol. However, when alcohol levels are high due to candida, low production of PAPS (sulfate deficiency) to buffer alcohol we make in metabolism, or alcohol intake, then the normal acid we make in metabolism in small amounts (or in large amounts if we are pantothenic acid deficient), acetic acid, becomes a catalyst to make AR. I’ll be posting more about this metabolite of vitamin A because I believe this is THE SNEAKY version of vitamin A that causes “vitamin A toxicity.” Below is a “road map” of anhydroretinol. You can start at the green arrows and work your way through the map, you can look at the blue ovals which are contributing factors that can lead you into this pathway, or you can look at the yellow ovals to find symptoms or lab work that matches your own health issues. This is a work in progress and some is hypothetical, but what I am sure of is the damaging effects of AR on the individuals I care for is directly related to the pathological damage it does to energy metabolism.
If you would prefer to listen to me babble on and on about this topic, here is a video! Enjoy. Or just scroll on by and read to avoid my crazy rant about AR. Hahaha!
(At the end of this video there is a link to a correction video to show that beta-apo-10′- carotenol is NOT the carotenal that inhibits CYP26. This is actually beta-apo-13′-carotenone. It’s made from retinol, retinaldehyde, and retinoic acid during oxidative stress. It is an inhibitor of CYP26 and can cause accumulation of retinoic acid leading to feedback inhibition of RALDH. This will cause accumulation of retinaldehyde and possibly formation of A2E. Also, beta-apo-13′- carotene is an antagonist for specific RXR and can inhibit gene transcription. It seems to be not a good guy. It’s the reason why a low vitamin A diet that still provides retinoic acid from animal protein, could lead to a worsening health status over time. Beta-apo-10′-carotenol is made from BCO2 metabolism, and if you have a slow BCO1 enzyme, this is still a risk if it is not buffered with retinol. It can act in place of retinol in PKCdelta and pathologically turn on PDHC. )
So…I’m a dietitian, not a doctor. This blog isn’t medical advice, but only a guideline to share with your own personal healthcare provider. It isn’t intended to diagnose or treat a condition without supervision of your own health care practitioner. Please don’t make any changes to your diet, supplements, nutrition, or medications without talking with your own provider who knows you and your health needs.
That being said, please read on….it’s intriguing!
Why is AR so toxic?
AR’s toxicity is due to quantum mechanics. Yes, that’s right. I am not a quantum mechanics expert. I’m a dietitian, but the general gist of the problem is that when retinol becomes AR, it shifts the double bond orientation, and it no longer behaves like retinol.
Retinol plays a pivotal role in a signaling molecule called PKCδ signalosome. This signalosome consists of PKCδ, retinol, and cytochrome C of the electron transport chain. The PKCδ signalosome works to sense the energy level in the cell. When the cell doesn’t need energy anymore, PKCδ inhibits pyruvate dehydrogenase kinase 2 (PDK2). PDK2 usually removes a phosphate groups from Thiamine Pyrophosphate on E1 to make the enzyme pyruvate dehydrogenase complex (PDHC) inactive. PDHC converts pyruvate to acetyl CoA. Turning off PDHC helps to stop the influx of acetyl CoA into Krebs cycle and keeps the cell from creating too many electrons in the intermembrane space.
This regulation is crucial because if too many electrons accumulate, then they leak back through the mitochondrial membrane into the matrix and form superoxide. Superoxide causes a metabolic cascade that shuts down the Krebs cycle at the level of alpha-ketoglutarate. Alpha-ketoglutarate is exported from the mitochondria to the cytosol where GABA is made. GABA is a sort of “antioxidant” for the liver because it upregulates enzymes of that deal with reactive oxygen species. Typically this restores the mitochondria to normal, but when individuals have broken pathways involved in this mopping up of reactive oxygen species, they struggle with the damages that occur.
The problem with AR taking the place of retinol in the PKCδ signalosome is that AR pathologically turns on PDHC. It doesn’t shut off. This eventually leads to depletion of all the cofactors needed to support restoration of the cells redox balance. In addition to AR causing this issue, apo-carotenal can also take the place of retinol on PKCδ signalosome and cause the same issue. The authors of the quantum chemistry study on retinoids report that if there is enough fresh retinol in the cell to “buffer” AR or apo-carotenal, then the cell should just have a sort of energy boost with a prolonged “on” state of PDHC, but the question I have is, “Can all people tolerate this prolonged on state or is this the start of an endless pathological cycle leading to more and more AR production within the body in those with broken redox pathways?”
AR toxicity may be due to the fact that it can’t be stored like retinol in hepatic stellate cells.
AR lacks the “-OH” group required for lecithin:retinol acyltransferase (LRAT) reaction using phosphatidylcholine to make a retinyl ester. LRAT is an enzyme that helps to package retinol into cellular retinol binding proteins and also onto retinol binding protein four (RPB4). Our cells have two back up enzymes, acyl-CoA:retinol acyltransferase (ARAT) and Acyl CoA: Diacylglycerol O-acyltransferase 1 (DGAT). These enzymes also require anhydroretinol to have an “-OH” group to interact with their “CoA” to bind a fat to retinol. ARAT and DGAT are back up enzymes for taking care of high levels of retinol in the cell. The acyl CoA portion of the fat they want to add requires pantothenic acid. I believe that AR causes pantothenic acid deficiency by pushing the conversion of pyruvate to acetyl CoA in excess. Interestingly, knockout of DGAT results in mice having alopecia. Many people with vitamin A toxicity experience alopecia. I’ve seen people lose parts of their eyebrows or have patches of hair missing from their heads. If DGAT is tied up in trying to bind retinol or rehydroretinol due to levels are too high in cells (a high NADH state prevents metabolism of retinol and would also prevent metabolism of rehydroretinol), then it’s busy mopping up retinol. That may be the cause of the alopecia, although a functional B6 deficiency is possible as well. The fact that AR can’t be stored, and that alcoholics have been found to have no storage of vitamin A in their liver, makes me highly suspicious that their alcohol intake plus the acetic acid or other possible acids in metabolism pushed them into the process of making AR from retinol.
How do we “detox” AR?
The fact that AR can’t be stored in hepatic stellate cells, means we have to get it out of liver cells. I have a sneaky suspicion that AR is “stored” in PKCδ, but that is yet to be explored as AR research is mostly limited to trying to induce cell death and it’s thought that it’s a very small part of vitamin A metabolism in vivo. However, I suspect AR production is significant in individuals with broken metabolic pathways. Children with MBD5 deletion in particular have reduced glutathione production. Genetics likely plays a huge role in susceptibility to AR toxicity.
AR can’t be stored like retinol in hepatic stellate cells because it lacks a polar “-OH” group, and this feature also may make it unable to diffuse through cell membranes. However, it has been found to have a high affinity for RBP4 and CRBP. This would, in theory ,displace retinol from RBP4 and also CRBP leaving healthy retinol unbound. This cause a high primary alcohol state. PAPS is a buffer for primary alcohols, like ethanol, but there is no sulfotransferase available for retinol, and so retinol will remain free in the cytosol.
I think it’s probable that we have to get into a state of “low alcohol” in a cell to be able to use an acid to convert AR to rehydroretinol. I also think it’s possible that if there is unbound retinol in the cell, it can be the “alcohol” that is causing the problem of making more retinol. This means that having plenty of phosphatidylcholine available for LRAT to safely tuck away vitamin A is important. The back up enzymes ARAT and DGAT are also helpful, but I suspect these are tied up in fatty acid synthesis as AR pushes people into ketosis and also makes them produce excessive lipids from citrate exportation from the cells. It is also possible that the low vitamin A diet can help someone get into a low retinol state. However, my own person experience with my daughter over the past three years was that a low vitamin A diet did not solve her vitamin A toxicity, and in fact she worsened. Hind sight tells me that this could be due to the lack of unadulterated retinol to buffer anhydroretinol in the cells.
Cellular control of retinol levels is crucial to prevent cell toxicity. The ability to excrete retinol from cells onto RBP4 to keep cytosolic levels of retinol normal is highly dependent on blood pH. In acidosis, calcium channels function poorly and calcium influx into cells decreases. Calcium is crucial for the STRA6 pore that allows retinol to leave the cell to open. Calcium actually binds to calmodulin, and when calcium levels increase in the cell, STRA6 doors are open. Unfortunately, when AR takes the place of retinol in PKCδ and pathologically turns on PDHC, the ensuing reactive oxygen species pushes cells into reluctant ketosis which causes acidosis. Thus, efflux of retinol from cells is stagnant, and until acidosis is resolved, retinol levels will stay high in cells, or if the cell is in a low retinol state, the levels will stay low. It may be though, since retinol is polar, that it can diffuse out of the cell through membranes. This remains to be explored, but it is possible that while retinol is doing this, which isn’t very efficient at all.
What if most of the retinol in the body is AR or Rehydroretinol?
The possibility that someone is so very far stuck in this pathway that they have no true retinol is quite possible. What would this look like? An individual that lacks sufficient, true retinol would have immune dysfunction. Blood work would show IgA and/or IgG deficiency. Stool testing would show IgA deficiency. This is due to retinoic acid is needed for the immune system to function. A person with severe AR toxicity might have low lymphocyte counts and high liver enzymes. I suspect this person’s underlying issue would be candida of the stomach that hasn’t been diagnosed. This would lead to all oral intakes of vitamin A being converted into anhydroretinol while in the gut. Someone with mild AR toxicity might only have low platelet counts and high monocytes as retinoic acid is needed to induce platelet production and it is needed to convert monocytes into macrophages. Of course, these are general ideas based on experience and reading individual labs of the people I care for.
What made me find AR as the sneaky vitamin A toxin?
After an entire year dealing with clients suffering from vitamin A toxicity as well as my own daughter having suffered for three years, one of my clients, Oasis, who has a CAPZB variant showed me that the true problem is anhydroretinol. His mother and I have become good friends and we prayed that we would find what is causing Oasis, Zoey, and my other client’s inability to use vitamin A correctly in their body. Our prayers were answered.
Oasis’ CAPZB variant makes him have an altered versions of F-Actin which is a cytoskeletal protein that gives membranes stability. He is more vulnerable to anything that causes oxidative stress to membranes. He has suffered greatly over the past five years from anhydroretinol toxicity from a combination of enteral nutrition, valproic acid altering his ability to bind retinol in the body, and altered metabolism. We can all thank Oasis and his mom Marcela for helping us to solve the puzzle of vitamin A toxicity, but not just them!
I also need to thank a few other clients and parents for trusting me in this deep dive. Olivia’s labs (in addition to Oasis’) showed me all the worst broken pathways when vitamin A is out of control. My own daughter Zoey’s urinary organic acid tests and plasma amino acid tests were quite revealing. The trends were consistent among these individuals. And finally, James, who has down syndrome, also helped me to find what’s truly going on in Vitamin A toxicity. There are a few other clients that are no longer with me due to a change in my employment, but they also helped me along this journey to find that ALL ROADS LEAD TO ANHYDRORETINOL.
Of course, I can’t forget my excellent sounding board that consisted of Andrew Baird, vitamin A researcher, Jenny Jones, PhD, molecular genetics, Michelle Harris, nutrition student (close to graduation!), and Bryan Toh, pharmacist. Without them checking my hypotheses and feeding bits of information that I didn’t know, because, yes, we all can’t know everything, I wouldn’t have finally found the root cause of vitamin A toxicity.
What is the rescue? How do we escape from the devastating effects of AR?
(Please consult with your personal healthcare provider before making changes)
In short, okay, not so short, the rescue is:
- Choline from phosphatidylcholine or eggs (I prefer free range eggs) – 500 mg of choline is the goal.
- Meeting at minimum the RDA for minerals especially Cu, Mo, Zn, Se, Mn. (may need more or less)
- Benfotiamine (down regulates NF-KB and lowers iNOS, restores pentose phosphate shunt generation of NADPH needed for glutathione recycling)
- Pantothenic acid (avoidance of biotin or lipoate at the same time as pantothenic acid; helps to restore acetate to acetyl CoA instead of becoming acetone). I am unsure of the amount. I am giving my own daughter 25 mg per day.
- Vitamin E (Possibly 400 IU per day. I don’t recommend going very high because we need enough vitamin C in the body to recycle the oxidized form of vitamin E.)
- Vitamin C (preferably slow release vitamin C, 250 mg per day, or dietary sources of vitamin C)
- Coenzyme Q10 (to mop up the excess electrons being produced in the cells from pathological turning on of PDHC)
- B12 – 2000 mcg per day
- Folate in the form of 5-methylfolate or folinic acid (although folinic acid may contribute to gout)
- After making sure mineral are restored, increase betaine intake or start betaine (trimethylglycine) but only 250 mg for kids and 500 mg for adults if you have not had a plasma amino acid tests to check methionine levels. There is a small chance that people in this pathway have high slow CBS enzymes which can cause high methionine levels and adding betaine could cause brain edema. This is very rare, but any children that are non-speaking should have a plasma amino acid test prior to starting betaine and never go over 1000 mg per day.
- At least 2 mg of riboflavin to support betaine pathway
- A source of retinol daily. I believe that many people who are making too much anhydroretinol could just have rehydroretinol available, and this version of vitamin A has altered double bond configuration and probably will not function like retinol (researchers say 7% activity of retinol). One source of retinol could be the eggs – many don’t agree with me on this, but I have found that eggs have rescued my clients from high AST and ALT. Also, eggs are a good source of biotin and biotin is needed for the STRA6 pore to work properly. Taking a biotin supplement is problematic because it competes with pantothenic acid for absorption. Another possible source of retinol is raw milk or butter (be sure to follow food safety precautions and find a reputable source).
- CAUTION with increase retinol in those with candida or other fungal infections. I think these people are the individuals who feel sick from consuming retinol sources. Treating fungal infections may be necessary before increasing retinol intake.
- Treatment of candida (There is a risk for candida of the esophagus and stomach in anyone who takes antihistamines. This may a contributor to making AR in the intestinal track before absorption. This is what is happening to my daughter.)
- Epsom salt baths to help PAPS (exploring PAPS due to APS pyruvate carboxylase)
- Liposomal glutathione. This is controversial because it’s possible that the glutathione is in a oxidized form. However, oxidized glutathione actually triggers the CBS enzyme to upregulate and make more glutathione. Also, with the restoration of the pentose phosphate shunt pathway using benfotiamine, there should be plenty of NADPH to be able to restore glutathione to its reduced state.
- Monitor for acidosis with health care practitioner and treat if able
- AVOID citrate supplements or citrate versions of supplements
- Preferable potassium bicarbonate or sodium bicarbonate (but not with meals)
- acidosis needs to be resolved because “first pass” of fresh retinol that is needed by cells in the peripheral tissues is dependent on uptake of triglycerides from chylomicron first.
Things to avoid if you are susceptible to anhydroretinol toxicity…
- Saccharomyces boulardii. This is an “anti-yeast” that people take when having candida. It is sometimes added to probiotics. This has been shown to make more alcohol than Candida
- Alcohol – alcohol is the determinant factor in making anhydroretinol in the gut. Especially never eat cheese with your alcohol!
- Spore based bacillus subtilis (metabolizes sulfate) and we actually need all the sulfate we can get to restore PAPS which takes care of excess alcohol in the cell. It won’t fix the high retinol problem. More on that in a later post.
- Annatto and Saffron – contain apo-carotenal and this could be pathological if in PKCδ
- Excess carotenoids if you KNOW your have a slow BCO1 because BCO2 makes various apo-carotenols which might displace retinol from PKCdelta.
- One carotenol that is made from retinol, retinaldehyde, and retinoic acid, under oxidative stress is beta-apo-13′-carotenal that is known to inhibit CYP26 and also likely turns on PDCH pathologically. (doi: 10.3390/nu14071411;
- “Shelf stable” foods with vitamin A added. Vitamin A degrades over time and the vitamin A added could have some anhydroretinol in it.
- Skin products with retinyl palmitate or retinol added as the UV light makes anhydroretinol in skin.
- Milk in clear containers. Avoid fat free milk that is fortified with vitamin A.
- Cod liver oil. This was the first product in which scientists found anhydroretinol!
- Any competitors for aldehyde oxidase. Aldehyde oxidase is the only enzyme to convert retinaldehyde to retinoic acid without requiring NAD. It is Mo dependent). Other substrates for AOX include niacin, caffeine, and vitamin B6. Often B6 levels will be high in the blood and that is a sneaky sign of having issues with anhydroretinol.
THE MASSIVE AMOUNT OF ARTICLES THAT I READ TO COME TO THIS CONCLUSION
- Hammerling U, Kim YK, Quadro L. Quantum chemistry rules retinoid biology. Commun Biol. 2023 Feb 28;6(1):227. doi: 10.1038/s42003-023-04602-x. PMID: 36854887; PMCID: PMC9974979.
- Hammerling U. Retinol as electron carrier in redox signaling, a new frontier in vitamin A research. Hepatobiliary Surg Nutr. 2016 Feb;5(1):15-28. doi: 10.3978/j.issn.2304-3881.2016.01.02. PMID: 26904553; PMCID: PMC4739943.
- Buck J, Grün F, Derguini F, Chen Y, Kimura S, Noy N, Hämmerling U. Anhydroretinol: a naturally occurring inhibitor of lymphocyte physiology. J Exp Med. 1993 Aug 1;178(2):675-80. doi: 10.1084/jem.178.2.675. PMID: 8340762; PMCID: PMC2191109.
- Acin-Perez R, Hoyos B, Zhao F, Vinogradov V, Fischman DA, Harris RA, Leitges M, Wongsiriroj N, Blaner WS, Manfredi G, Hammerling U. Control of oxidative phosphorylation by vitamin A illuminates a fundamental role in mitochondrial energy homoeostasis. FASEB J. 2010 Feb;24(2):627-36. doi: 10.1096/fj.09-142281. Epub 2009 Oct 7. PMID: 19812372; PMCID: PMC2812036.
- Kim YK, Hammerling U. The mitochondrial PKCδ/retinol signal complex exerts real-time control on energy homeostasis. Biochim Biophys Acta Mol Cell Biol Lipids. 2020 Nov;1865(11):158614. doi: 10.1016/j.bbalip.2020.158614. Epub 2020 Jan 10. PMID: 31927141; PMCID: PMC7347429.
- Korichneva I, Hämmerling U. F-actin as a functional target for retro-retinoids: a potential role in anhydroretinol-triggered cell death. J Cell Sci. 1999 Aug;112 ( Pt 15):2521-8. doi: 10.1242/jcs.112.15.2521. PMID: 10393808.
- Md. Jakaria, Abdel A. Belaidi, Ashley I. Bush, Scott Ayton, Vitamin A metabolites inhibit ferroptosis [anhydroretinol sensitizes cells to ferroptosis], Biomedicine & PharOasistherapy, Volume 164, 2023, 114930, ISSN 0753-3322, https://doi.org/10.1016/j.biopha.2023.114930.
- T.K. Murray, P. Erdody, The absorption and metabolism of anhydrovitamin A by the rat, Biochimica et Biophysica Acta (BBA) – General Subjects, Volume 136, Issue 2, 1967, Pages 375-378, ISSN 0304-4165, https://doi.org/10.1016/0304-4165(67)90082-7.
- Martin Kohlmeier,Chapter 9 – Fat-Soluble Vitamins and Nonnutrients,Editor(s): Martin Kohlmeier,Nutrient Metabolism (Second Edition),Academic Press,2015,Pages479-565,ISBN 9780123877840,https://doi.org/10.1016/B978-0-12-387784-0.00009-2.
- Gloria E. Mao, Michael D. Collins, Fadila Derguini, Teratogenicity, Tissue Distribution, and Metabolism of the retro-Retinoids, 14-Hydroxy-4,14-retro-retinol and Anhydroretinol, in the C57BL/6J Mouse,Toxicology and Applied PharOasislogy,Volume 163, Issue 1,2000,Pages 38-49,ISSN 0041-008X, https://doi.org/10.1006/taap.1999.8828.
- O’Connell MJ, Chua R, Hoyos B, Buck J, Chen Y, Derguini F, Hämmerling U. Retro-retinoids in regulated cell growth and death. J Exp Med. 1996 Aug 1;184(2):549-55. doi: 10.1084/jem.184.2.549. PMID: 8760808; PMCID: PMC2192720.
- Chen Y, Buck J, Derguini F. Anhydroretinol induces oxidative stress and cell death. Cancer Res. 1999 Aug 15;59(16):3985-90. PMID: 10463596.
- Chiu HJ, Fischman DA, Hammerling U. Vitamin A depletion causes oxidative stress, mitochondrial dysfunction, and PARP-1-dependent energy deprivation. FASEB J. 2008 Nov;22(11):3878-87. doi: 10.1096/fj.08-112375. Epub 2008 Aug 1. PMID: 18676402; PMCID: PMC2574026.
- de Oliveira MR. Vitamin A and Retinoids as Mitochondrial Toxicants. Oxid Med Cell Longev. 2015;2015:140267. doi: 10.1155/2015/140267. Epub 2015 May 19. PMID: 26078802; PMCID: PMC4452429.
- Klamt F, Dal-Pizzol F, Gelain DP, Dalmolin RS, Birnfeld de Oliveira R, Bastiani M, Horn F, Fonseca Moreira JC. Vitamin A treatment induces apoptosis through an oxidant-dependent activation of the mitochondrial pathway. Cell Biol Int. 2008 Jan;32(1):100-6. doi: 10.1016/j.cellbi.2007.08.018. Epub 2007 Sep 7. PMID: 17942326.
- Álvarez, R., Vaz, B., Gronemeyer, H., & de Lera, A.R. (2014). Functions, therapeutic applications, and synthesis of retinoids and carotenoids. Chemical reviews, 114 1, 1-125 .
- Yokoyama H, Matsumoto M, Shiraishi H, Miyagi M, Kato And S, Ishii H. Nicotinamide adenine dinucleotide-dependent retinoic acid formation from retinol in the human gastric mucosa: inhibition by ethanol, acetaldehyde, and H2 blockers. Alcohol Clin Exp Res. 2001 Jun;25(6 Suppl):24S-8S. doi: 10.1097/00000374-200106001-00007. PMID: 11410737.
- Ramkumar S, Moon J, Golczak M, von Lintig J. LRAT coordinates the negative-feedback regulation of intestinal retinoid biosynthesis from β-carotene. J Lipid Res. 2021;62:100055. doi: 10.1016/j.jlr.2021.100055. Epub 2021 Feb 23. PMID: 33631212; PMCID: PMC8010212.
- Bhat PV, Roller PP, De Luca LM. Chemical and biological studies on 5,6-epoxyretinol, retinol, and their phosphoryl esters. J Lipid Res. 1981 Sep;22(7):1069-78. PMID: 7299288.
- Zweier, Jay & Velayutham, Murugesan & Hemann, Craig. (2013). FRBM4.
- Tejada-Jimenez M, Chamizo-Ampudia A, Calatrava V, Galvan A, Fernandez E, Llamas A. From the Eukaryotic Molybdenum Cofactor Biosynthesis to the Moonlighting Enzyme mARC. Molecules. 2018; 23(12):3287. https://doi.org/10.3390/molecules23123287
- Wang CH, Zhang C, Xing XH. Xanthine dehydrogenase: An old enzyme with new knowledge and prospects. Bioengineered. 2016 Nov;7(6):395-405. doi: 10.1080/21655979.2016.1206168. Epub 2016 Aug 18. PMID: 27537049; PMCID: PMC5094624.
- Jansson EA, Huang L, Malkey R, Govoni M, Nihlén C, Olsson A, Stensdotter M, Petersson J, Holm L, Weitzberg E, Lundberg JO. A mammalian functional nitrate reductase that regulates nitrite and nitric oxide homeostasis. Nat Chem Biol. 2008 Jul;4(7):411-7. doi: 10.1038/nchembio.92. Epub 2008 May 30. PMID: 18516050.
- Gudz TI, Tserng KY, Hoppel CL. Direct inhibition of mitochondrial respiratory chain complex III by cell-permeable ceramide. J Biol Chem. 1997 Sep 26;272(39):24154-8. doi: 10.1074/jbc.272.39.24154. PMID: 9305864.
- Zeczycki TN, Maurice MS, Attwood PV. Inhibitors of Pyruvate Carboxylase. Open Enzym Inhib J. 2010;3:8-26. doi: 10.2174/1874940201003010008. PMID: 22180764; PMCID: PMC3238542.
- Moffett JR, Puthillathu N, Vengilote R, Jaworski DM, Namboodiri AM. Acetate Revisited: A Key Biomolecule at the Nexus of Metabolism, Epigenetics and Oncogenesis-Part 1: Acetyl-CoA, Acetogenesis and Acyl-CoA Short-Chain Synthetases. Front Physiol. 2020 Nov 12;11:580167. doi: 10.3389/fphys.2020.580167. PMID: 33281616; PMCID: PMC7689297.
- Gray LR, Tompkins SC, Taylor EB. Regulation of pyruvate metabolism and human disease. Cell Mol Life Sci. 2014 Jul;71(14):2577-604. doi: 10.1007/s00018-013-1539-2. Epub 2013 Dec 21. PMID: 24363178; PMCID: PMC4059968.
- Velayutham M, Hemann CF, Cardounel AJ, Zweier JL. Sulfite Oxidase Activity of Cytochrome c: Role of Hydrogen Peroxide. Biochem Biophys Rep. 2016 Mar 1;5:96-104. doi: 10.1016/j.bbrep.2015.11.025. PMID: 26709389; PMCID: PMC4689149.
- Kundu TK, Velayutham M, Zweier JL. Aldehyde oxidase functions as a superoxide generating NADH oxidase: an important redox regulated pathway of cellular oxygen radical formation. Biochemistry. 2012 Apr 3;51(13):2930-9. doi: 10.1021/bi3000879. Epub 2012 Mar 19. PMID: 22404107; PMCID: PMC3954720.
- Kitamura S, Sugihara K, Ohta S. Drug-metabolizing ability of molybdenum hydroxylases. Drug Metab Pharmacokinet. 2006 Apr;21(2):83-98. doi: 10.2133/dmpk.21.83. PMID: 16702728.
- https://owl.oit.umass.edu/departments/OrganicChemistry/appendix/pKaTable.html
- Appendix C: Dissociation Constants and pKa Values for Acids at 25°C”, appendix 3 from the book Principles of General Chemistry (v. 1.0).
- Tolleson WH, Cherng SH, Xia Q, Boudreau M, Yin JJ, Wamer WG, Howard PC, Yu H, Fu PP. Photodecomposition and phototoxicity of natural retinoids. Int J Environ Res Public Health. 2005 Apr;2(1):147-55. doi: 10.3390/ijerph2005010147. PMID: 16705812; PMCID: PMC3814709.
- Álvarez, R., Vaz, B., Gronemeyer, H., & de Lera, A.R. (2014). Functions, therapeutic applications, and synthesis of retinoids and carotenoids. Chemical reviews, 114 1, 1-125 .
- Kinetics of Formation of Anhydroretinol From Retinyl Acetate in Acetic Acid, and From Retinyl Acetate and Retinol in Ethanol and Ethanol–Water Mixtures Containing Hydrogen Chloride.Acta Chemica Scandinavicadoi 10.3891/acta.chem.scand.30a-0285
- Saeed A, Bartuzi P, Heegsma J, Dekker D, Kloosterhuis N, de Bruin A, Jonker JW, van de Sluis B, Faber KN. Impaired Hepatic Vitamin A Metabolism in NAFLD Mice Leading to Vitamin A Accumulation in Hepatocytes. Cell Mol Gastroenterol Hepatol. 2021;11(1):309-325.e3. doi: 10.1016/j.jcmgh.2020.07.006. Epub 2020 Jul 19. PMID: 32698042; PMCID: PMC7768561.
- Ciorba MA. Kynurenine pathway metabolites: relevant to vitamin B-6 deficiency and beyond. Am J Clin Nutr. 2013 Oct;98(4):863-4. doi: 10.3945/ajcn.113.072025. Epub 2013 Aug 28. PMID: 23985806; PMCID: PMC4498264.
- Sherriff JL, O’Sullivan TA, Properzi C, Oddo JL, Adams LA. Choline, Its Potential Role in Nonalcoholic Fatty Liver Disease, and the Case for Human and Bacterial Genes. Adv Nutr. 2016 Jan 15;7(1):5-13. doi: 10.3945/an.114.007955. PMID: 26773011; PMCID: PMC4717871.
- Harrison EH, Quadro L. Apocarotenoids: Emerging Roles in Mammals. Annu Rev Nutr. 2018 Aug 21;38:153-172. doi: 10.1146/annurev-nutr-082117-051841. Epub 2018 May 11. PMID: 29751734; PMCID: PMC6295211.
- Fortuna VA, Trugo LC, Borojevic R. Acyl-CoA: retinol acyltransferase (ARAT) and lecithin:retinol acyltransferase (LRAT) activation during the lipocyte phenotype induction in hepatic stellate cells. J Nutr Biochem. 2001 Nov;12(11):610-621. doi: 10.1016/s0955-2863(01)00179-6. PMID: 12031254.
- O’Byrne SM, Blaner WS. Retinol and retinyl esters: biochemistry and physiology. J Lipid Res. 2013 Jul;54(7):1731-43. doi: 10.1194/jlr.R037648. Epub 2013 Apr 26. PMID: 23625372; PMCID: PMC3679378.
- Shih MY, Kane MA, Zhou P, Yen CL, Streeper RS, Napoli JL, Farese RV Jr. Retinol Esterification by DGAT1 Is Essential for Retinoid Homeostasis in Murine Skin. J Biol Chem. 2009 Feb 13;284(7):4292-9. doi: 10.1074/jbc.M807503200. Epub 2008 Nov 20. PMID: 19028692; PMCID: PMC2640966.
- Lillig, Christopher Horst & Fernandes, Aristi & Schwenn, Jens & Vlamis-Gardikas, Alexios & Holmgren, Arne. (2003). Redox Regulation of 3′-Phosphoadenylylsulfate Reductase from Escherichia coli by Glutathione and Glutaredoxins. The Journal of biological chemistry. 278. 22325-30. 10.1074/jbc.M302304200.
- Carnauba RA, Baptistella AB, Paschoal V, Hübscher GH. Diet-Induced Low-Grade Metabolic Acidosis and Clinical Outcomes: A Review. Nutrients. 2017 May 25;9(6):538. doi: 10.3390/nu9060538. PMID: 28587067; PMCID: PMC5490517.
- Yamada K, Yoshida K. Multiple subcellular localizations and functions of protein kinase Cδ in liver cancer. World J Gastroenterol. 2022 Jan 14;28(2):188-198. doi: 10.3748/wjg.v28.i2.188. PMID: 35110944; PMCID: PMC8776529.
- Boer, Johannes & Alsters, Paul & Meetsma, Auke & Hage, Ronald & Browne, Wesley & Feringa, Ben. (2008). The role of salicylic acid, L-ascorbic acid and oxalic acid in promoting the oxidation of alkenes with H2O2 catalysed by [MnIV2(O)3(tmtacn)2]2+. Dalton transactions (Cambridge, England : 2003). 44. 6283-95. 10.1039/b809177c.
- Webster CR, Johnston AN, Anwer MS. Protein kinase Cδ protects against bile acid apoptosis by suppressing proapoptotic JNK and BIM pathways in human and rat hepatocytes. Am J Physiol Gastrointest Liver Physiol. 2014 Dec 15;307(12):G1207-15. doi: 10.1152/ajpgi.00165.2014. Epub 2014 Oct 30. PMID: 25359536; PMCID: PMC4269680.
- Leitges, Michael & Gimborn, Kerstin & Elis, Winfried & Kalesnikoff, Janet & Hughes, Michael & Krystal, Gerald & Huber, Michael. (2002). Protein Kinase C- Is a Negative Regulator of Antigen-Induced Mast Cell Degranulation. Molecular and cellular biology. 22. 3970-80. 10.1128/MCB.22.12.3970-3980.2002.
- Penniston, Kristina & Tanumihardjo, Sherry. (2006). The acute and chronic effects of vitamin A. The American journal of clinical nutrition. 83. 191-201. 10.1093/ajcn/83.2.191.
- O’Connell MJ, Chua R, Hoyos B, Buck J, Chen Y, Derguini F, Hämmerling U. Retro-retinoids in regulated cell growth and death. J Exp Med. 1996 Aug 1;184(2):549-55. doi: 10.1084/jem.184.2.549. PMID: 8760808; PMCID: PMC2192720.
- Gimeno A, Zaragozá R, Vivó-Sesé I, Viña JR, Miralles VJ. Retinol, at concentrations greater than the physiological limit, induces oxidative stress and apoptosis in human dermal fibroblasts. Exp Dermatol. 2004 Jan;13(1):45-54. doi: 10.1111/j.0906-6705.2004.00112.x. PMID: 15009115.
- Venkatachalam, Kallidaikurichi. (2015). Biochemical Sulfuryl Group Transfer From 3’-Phosphoadenosine 5’-Phosphosulfate (PAPS) Versus Phosphoryl Transfer From ATP: What Can Be Learnt?. Biochemistry and Physiology. 5. 10.4172/2168-9652.1000192.
- MAHTAB S. BAMJI, H. R. CAMA, AND P. R. SUNDARESAN. THE JOURNAL CIBOLO(ICAL CHEMISTRY Anhydrovitamin A, and Rehydrovitamin A,. II Vol. 237, No. 9,September 1962 Printed in U.S.A.
- P. Phuapradit, M.R. Lakshmanan, J.A. Olson,The excretion of polar metabolites of radioactive anhydroretinol in rabbit bile,Biochimica et Biophysica Acta (BBA) – Lipids and Lipid Metabolism,Volume 260, Issue 4,1972,Pages 666-669,ISSN 0005-2760, https://doi.org/10.1016/0005-2760(72)90015-X.
- Philip D. Kiser, Marcin Golczak, and Krzysztof Palczewski Chemistry of the Retinoid (Visual) Cycle Chemical Reviews 2014 114 (1), 194-232 DOI: 10.1021/cr400107q
- Minren Xu and Jim WatsonMicroencapsulated Vitamin A Palmitate Degradation Mechanism Study To Improve the Product Stability Journal of Agricultural and Food Chemistry 2021 69 (51), 15681-15690 DOI: 10.1021/acs.jafc.1c06087
- Yang H, Xu L, Hou L, Xu TC, Ye SH. Stability of vitamin A, E, C and thiamine during storage of different powdered enteral formulas. Heliyon. 2022 Nov 12;8(11):e11460. doi: 10.1016/j.heliyon.2022.e11460. PMID: 36411896; PMCID: PMC9674494.
- Scientific Opinion on the safety and efficacy of vitamin A (retinyl acetate, retinyl palmitate and retinyl propionate) as a feed additive for all animal species and categories EFSA Panel on Additives and Products or Substances used in Animal Feed (FEEDAP) First published: 08 January 2013 https://doi.org/10.2903/j.efsa.2013.3037
- Mao GE, Collins MD, Derguini F. Teratogenicity, tissue distribution, and metabolism of the retro-retinoids, 14-hydroxy-4,14-retro-retinol and anhydroretinol, in the C57BL/6J mouse. Toxicol Appl PharOasisl. 2000 Feb 15;163(1):38-49. doi: 10.1006/taap.1999.8828. PMID: 10662603.
- Zhong G, Seaman CJ, Paragas EM, Xi H, Herpoldt KL, King NP, Jones JP, Isoherranen N. Aldehyde Oxidase Contributes to All-Trans-Retinoic Acid Biosynthesis in Human Liver. Drug Metab Dispos. 2021 Mar;49(3):202-211. doi: 10.1124/dmd.120.000296. Epub 2020 Dec 18. PMID: 33355213; PMCID: PMC7885020.
- Modin A, Björne H, Herulf M, Alving K, Weitzberg E, Lundberg JO. Nitrite-derived nitric oxide: a possible mediator of ‘acidic-metabolic’ vasodilation. Acta Physiol Scand. 2001 Jan;171(1):9-16. doi: 10.1046/j.1365-201X.2001.00771.x. PMID: 11350258.
- Flores-Cortez YA, Barragán-Bonilla MI, Mendoza-Bello JM, González-Calixto C, Flores-Alfaro E, Espinoza-Rojo M. Interplay of retinol binding protein 4 with obesity and associated chronic alterations (Review). Mol Med Rep. 2022 Jul;26(1):244. doi: 10.3892/mmr.2022.12760. Epub 2022 Jun 3. PMID: 35656886; PMCID: PMC9185696.
- Bozic I, Savic D, Laketa D, Bjelobaba I, Milenkovic I, Pekovic S, Nedeljkovic N, Lavrnja I. Benfotiamine attenuates inflammatory response in LPS stimulated BV-2 microglia. PLoS One. 2015 Feb 19;10(2):e0118372. doi: 10.1371/journal.pone.0118372. PMID: 25695433; PMCID: PMC4335016.
- Veselá A, Wilhelm J. The role of carbon dioxide in free radical reactions of the organism. Physiol Res. 2002;51(4):335-9. PMID: 12449430.
- Ciorba MA. Kynurenine pathway metabolites: relevant to vitamin B-6 deficiency and beyond. Am J Clin Nutr. 2013 Oct;98(4):863-4. doi: 10.3945/ajcn.113.072025. Epub 2013 Aug 28. PMID: 23985806; PMCID: PMC4498264.
- Robert Harker DM, Martinez B, Tabaac BJ. B12 Deficiency and Clinical Presentation in the Setting of Nitric Oxide Use. Case Rep Neurol Med. 2021 Apr 8;2021:5590948. doi: 10.1155/2021/5590948. PMID: 33927908; PMCID: PMC8049814.
- Amiraslani B, Sabouni F, Abbasi S, Nazem H, Sabet M. Recognition of betaine as an inhibitor of lipopolysaccharide-induced nitric oxide production in activated microglial cells. Iran Biomed J. 2012;16(2):84-9. doi: 10.6091/ibj.1012.2012. PMID: 22801281; PMCID: PMC3600952.
- Nicolaou A, Kenyon SH, Gibbons JM, Ast T, Gibbons WA. In vitro inactivation of mammalian methionine synthase by nitric oxide. Eur J Clin Invest. 1996 Feb;26(2):167-70. doi: 10.1046/j.1365-2362.1996.122254.x. PMID: 8904527.
- Sharma VS, Pilz RB, Boss GR, Magde D. Reactions of nitric oxide with vitamin B12 and its precursor, cobinamide. Biochemistry. 2003 Jul 29;42(29):8900-8. doi: 10.1021/bi034469t. PMID: 12873151.
- Broderick KE, Singh V, Zhuang S, Kambo A, Chen JC, Sharma VS, Pilz RB, Boss GR. Nitric oxide scavenging by the cobalamin precursor cobinamide. J Biol Chem. 2005 Mar 11;280(10):8678-85. doi: 10.1074/jbc.M410498200. Epub 2005 Jan 4. PMID: 15632180.
- Danishpajooh IO, Gudi T, Chen Y, Kharitonov VG, Sharma VS, Boss GR. Nitric oxide inhibits methionine synthase activity in vivo and disrupts carbon flow through the folate pathway. J Biol Chem. 2001 Jul 20;276(29):27296-303. doi: 10.1074/jbc.M104043200. Epub 2001 May 22. PMID: 11371572.
- Sampaio AL, Dalli J, Brancaleone V, D’Acquisto F, Perretti M, Wheatley C. Biphasic modulation of NOS expression, protein and nitrite products by hydroxocobalamin underlies its protective effect in endotoxemic shock: downstream regulation of COX-2, IL-1β, TNF-α, IL-6, and HMGB1 expression. Mediators Inflamm. 2013;2013:741804. doi: 10.1155/2013/741804. Epub 2013 May 28. PMID: 23781123; PMCID: PMC3679756.
- Weinberg JB, Chen Y, Jiang N, Beasley BE, Salerno JC, Ghosh DK. Inhibition of nitric oxide synthase by cobalamins and cobinamides. Free Radic Biol Med. 2009 Jun 15;46(12):1626-32. doi: 10.1016/j.freeradbiomed.2009.03.017. Epub 2009 Mar 27. Erratum in: Free Radic Biol Med. 2011 Oct 1;51(7):1471. PMID: 19328848; PMCID: PMC2745708.
- Stincone A, Prigione A, Cramer T, Wamelink MM, Campbell K, Cheung E, Olin-Sandoval V, Grüning NM, Krüger A, Tauqeer Alam M, Keller MA, Breitenbach M, Brindle KM, Rabinowitz JD, Ralser M. The return of metabolism: biochemistry and physiology of the pentose phosphate pathway. Biol Rev Camb Philos Soc. 2015 Aug;90(3):927-63. doi: 10.1111/brv.12140. Epub 2014 Sep 22. PMID: 25243985; PMCID: PMC4470864.
- Young BD, Varney KM, Wilder PT, Costabile BK, Pozharski E, Cook ME, Godoy-Ruiz R, Clarke OB, Mancia F, Weber DJ. Physiologically Relevant Free Ca2+ Ion Concentrations Regulate STRA6-Calmodulin Complex Formation via the BP2 Region of STRA6. J Mol Biol. 2021 Nov 5;433(22):167272. doi: 10.1016/j.jmb.2021.167272. Epub 2021 Sep 27. PMID: 34592217; PMCID: PMC8568335.
- O’Connor C, Varshosaz P, Moise AR. Mechanisms of Feedback Regulation of Vitamin A Metabolism. Nutrients. 2022 Mar 21;14(6):1312. doi: 10.3390/nu14061312. PMID: 35334970; PMCID: PMC8950952.
- Harrison EH. Carotenoids, β-Apocarotenoids, and Retinoids: The Long and the Short of It. Nutrients. 2022 Mar 28;14(7):1411. doi: 10.3390/nu14071411. PMID: 35406024; PMCID: PMC9003029.
- Durojaye BO, Riedl KM, Curley RW Jr, Harrison EH. Uptake and metabolism of β-apo-8′-carotenal, β-apo-10′-carotenal, and β-apo-13-carotenone in Caco-2 cells. J Lipid Res. 2019 Jun;60(6):1121-1135. doi: 10.1194/jlr.M093161. Epub 2019 Mar 6. PMID: 30846527; PMCID: PMC6547639.